
Ametris Announces a New Suite of Digital Measures for Tracking Functional Capacity to Advance Neuromuscular Disease Research

|
Written by Rakesh Pilkar, PhD |
Ametris is excited to offer the novel Six-Minute Activity 95th Centile (6M95C)1 and the EMA qualified digital measure, Stride Velocity 95th Centile (SV95C) in the Ametris Platform for use in research and clinical trials in both ambulatory and non-ambulatory neuromuscular disorders.
Six-Minute Activity 95th Centile (6M95C)
Why measure 6M95C?
6M95C captures a patient’s peak movement over six minutes, providing a sensitive, objective, and effort-independent measure of skeletal muscle function. Unlike traditional tests, it works for both ambulatory and non-ambulatory patients, tracks real-world activity, and detects functional decline early. In the recently published study1, investigators showed that 6M95C, strongly correlates with muscle strength and can be used to track disease progression in patients with Duchenne muscular dystrophy (DMD). These metrics were able to differentiate ambulatory and non-ambulatory patients and reliably reflect functional decline over time. The 6M95C shows promise as an effort-independent, objective outcome measure for monitoring skeletal muscle progression in both ambulatory and non-ambulatory individuals, offering a powerful tool for clinical development and patient care.
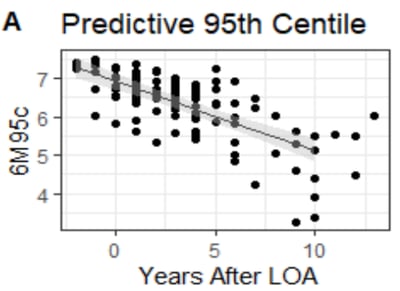
Figure A. The strong fit of the regression model predicting 6M95C based on time since loss of ambulation in DMD supports its use as an outcomes measure of skeletal myopathy progression1
How is 6M95C calculated?
Participants wear a wrist-worn accelerometer continuously, typically for seven days, with the device recording raw activity counts or acceleration in short epochs (e.g., 1-second or 1-minute intervals). The data are analyzed using rolling six-minute windows across the entire wear period, with the total activity (counts) calculated for each window. From all six-minute totals, the 95th percentile is computed, producing the six-minute activity-95th centile (6M95C), which reflects the level of activity higher than 95% of all windows and captures the patient’s higher spectrum of functional movement in daily life.
Stride Velocity 95th Centile (SV95C)
Why measure SV95C?
SV95C is a digital measure of mobility that represents the 95th percentile of stride velocities recorded during walking. This measurement reflects the upper range that an individual achieves while walking during their daily life, which is particularly relevant in understanding how the physical functioning of individuals with neurodegenerative or motor disorders changes over time and/or in response to treatment.
SV95C was qualified as a primary endpoint in clinical trials including Duchenne Muscular Dystrophy (DMD) patients aged ≥5 years by the EMA in 20192. In part, this qualification was supported by data that SV95C detected clinically meaningful changes of 6-12 months, even before loss of ambulation, in this patient population3.
SV95C is highly relevant in other neurodegenerative and motor disorders, such as Becker Muscular Dystrophy (BMD), Spinal Muscular Atrophy (SMA, ambulant types), Myotonic Dystrophy (DM1 and DM2), Spinal Bulbar Muscular Atrophy (SBMA) and Amyotrophic Lateral Sclerosis (ALS). Preliminary data that the Ametris team has collected with collaborators suggests that SV95C correlates with traditional clinical outcome assessments (COAs).
 Figure B. Correlation of 95C of stride velocities recorded during 6-minute walk
Figure B. Correlation of 95C of stride velocities recorded during 6-minute walk
with measured distance in six participants with SBMA.
How is SV95C calculated?
SV95C is calculated from continuous inertial measurement unit (IMU) sensor data collected from the ActiGraph LEAP® wearable device positioned on the ankle. The algorithm segments the walking strides from the raw data and calculates the velocity per stride. All the strides during a collection period are then aggregated to calculate 95th percentile. It is recommended to record ~180 hours of data for reliable SV95C measures.
SV95C gives researchers the opportunity to better understand how patients function in their daily lives, in addition to how they perform during in-clinic assessments. This digital measure is sensitive, objective, low-burden, and there is precedent for regulatory agencies accepting it as a clinical trial endpoint4, making it a very promising endpoint for clinical development in neurodegenerative conditions with mobility impairment.
Ametris’ ankle-based algorithm also provides additional gait measures of importance in this clinical population, such as step count, cadence, gait speed, and walking distance.
Comparison of SV95C and 6M95C
| Feature / Measure | SV95C (Stride Velocity 95th Centile) |
6M95C (Six-Minute Activity 95th Centile) |
| Regulatory Status | EMA-qualified for DMD | Novel, research-use measure (emerging validation) |
| Sensor Placement | Ankle-worn IMU (ActiGraph LEAP) | Wrist-worn accelerometer |
| Recommended Wear Period | ~180 hours (~2–3 weeks) | ~ 7 days |
| Construct | Fastest stride velocity during daily walking | Peak activity over six-minute windows in daily life |
| Concept of Interest | Mobility / Gait | Overall Activity |
| Patient Meaningfulness | High | High |
| Patient Applicability | Ambulatory | Ambulatory and non-ambulatory patients |
| Effort Dependence | Partially effort-dependent (walking) | Effort-independent |
| Sensitivity | Detects clinically meaningful changes in 6–12 months, even pre-ambulation loss | Detects early functional decline and tracks DMD progression |
| Strengths | EMA-qualified, validated against traditional clinical outcome assessments, sensitive to change | Works for all mobility levels, uses low-power requirements, higher compliance, early detection of functional decline |
| Limitations | Only applicable to ambulatory patients, higher battery consumption, possible low wear compliance | Novel measure, still emerging regulatory acceptance |
| Use Cases | Clinical trials in DMD, BMD, SMA, DM1/DM2, SBMA, ALS | |
Summary
SV95C and 6M95C are cutting-edge digital measures that capture peak mobility and activity in real-world settings, offering highly sensitive, patient-meaningful insights into disease progression in neuromuscular disorders. SV95C is EMA-qualified and tracks maximal stride velocity to detect early functional changes in ambulatory patients, while 6M95C provides an effort-independent measure of skeletal muscle function for both ambulatory and non-ambulatory individuals. Together, they enable objective, low-burden monitoring of functional decline and treatment effects, making them powerful endpoints for neuromuscular clinical trials and accelerating patient-centered research.
References
- Joy N, Soslow J, Burnette WB, Liu A, Guo CC, Pilkar R, Slaughter JC, Xu M, Crum K, Su K, Spurney C, Husain N, Gambetta K, Soriano BD, Raucci FJ Jr, Hor K, Markham LW, Tamaroff J; DMDCCC Investigators. Six-Minute Activity-95th Centile, a Novel Wearable-Derived Clinical Outcome Assessment for Duchenne Muscular Dystrophy. Pediatr Neurol. 2025 Nov 29;175:187-195. doi: 10.1016/j.pediatrneurol.2025.11.017. Epub ahead of print. PMID: 41401666.
- Committee for Medicinal Products for Human Use (CHMP), European Medicines Society, Qualification Opinion for Stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne Muscular Dystrophy studies, 28 July 2023, EMADOC-1700519818-1127132.
- Servais, L., Strijbos, P., Poleur, M. et al. Evidentiary basis of the first regulatory qualification of a digital primary efficacy endpoint. Sci Rep 14, 29681 (2024). Doi: 10.1038/s41598-024-80177-9
- Servais L, Yen K, Guridi M, Lukawy J, Vissière D, Strijbos P. Stride Velocity 95th Centile: Insights into Gaining Regulatory Qualification of the First Wearable-Derived Digital Endpoint for use in Duchenne Muscular Dystrophy Trials. J Neuromuscul Dis. 2022;9(2):335-346. doi: 10.3233/JND-210743. PMID: 34958044; PMCID: PMC9028650.
